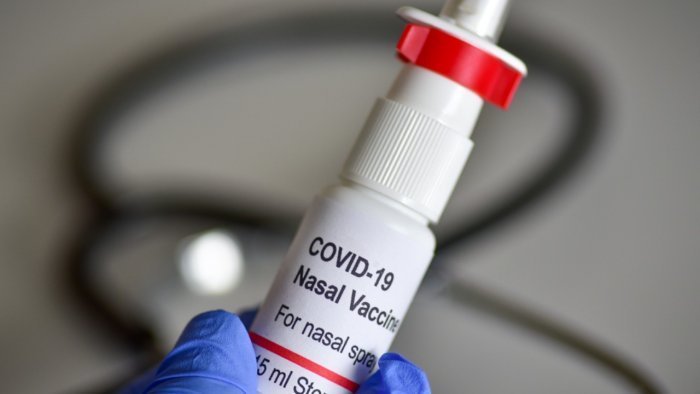
New Delhi, 28/1: The Drug Controller General of India (DCGI) has approved the trial of Bharat Biotech’s Nasal Vaccine. This vaccine will be used as a booster dose. At present, its trial will be done on 900 people. This will be the trial of the third dose of this vaccine. The company had sent the data for the trial to the subject expert committee of DCGI about 3 weeks ago. It is being said that this vaccine given through the nose will help people in the defense against Omicron.
It is being said that Bharat Biotech has proposed to use its nasal vaccine as a booster dose. This booster dose will be given to those who have previously taken the vaccine of Kovishield or Covaccine. Let us tell you that from this month in India, people working in the frontline are being given the third dose of corona vaccine.
On whom will the trial be held?
Bharat Biotech has proposed to use its nasal vaccine BBV154 as a booster dose in already vaccinated people. The clinical trial of the vaccine will be done on people who have already taken Kovashield or Covaccine.
Benefits of Nasal Vaccine
It is being said that the nasal vaccine is more effective than the common vaccine. According to doctors, when the vaccine is given through the nose, antibodies will first be formed in the nose. This will make it difficult for the virus to reach the lungs through breathing. The result will be that the virus will not be able to reach the lungs of those taking the nasal vaccine.
Comments are closed.